STATE OF THE ART
Fumaric acid is a widely distributed organic acid in nature and takes its name from the plant in which it was originally discovered, Fumaria Officinalis (Fam: PAPAVERACEAE). It is widely used in food and industry, extracted mainly from mushrooms of the genus Rhizopus Nigricans, Oryzae, etc. (Fam: MUCORACEAE) or synthesized by isomerization of maleic acid.
According to IUPAC nomenclature, it takes the name “Trans-Butenedioic acid” (p.m.= 116.07 g/mol).
It has two acid functions (pKa1 = 3.03 and pKa2 = 4.44) and two unsaturations at αand β position.
Only recently has its use in oenology been authorized by EU regulation 2022/68 dated 08/02/2022 for:
- Control the proliferation of lactic acid bacteria and malolactic fermentation (MLF).
- Promote, as an indirect effect, the preservation of malic acid.
- Adjuvant for reducing sulfur dioxide content in wines.
To date, the regulations do not provide guidance on either maximum allowable doses of use or repeated addition over time; instead, they indicate 30-60 g/HL as the range of doses sufficient to counteract a high lactic acid bacteria content or even capable of stopping an ongoing MLF.
Its use after alcoholic fermentation is complete is strongly recommended, as fumaric acid is involved, in all aerobic organisms, in the Krebs cycle. Reason being that Saccharomices Cerevisiae, the main yeast responsible for alcoholic fermentation, could metabolize fumaric acid and produce malic acid and succinic acid in different proportions resulting in the disappearance of fumaric acid and its effectiveness.
In terms of its chemical and physical properties, it could be compared to tartaric acid, the main organic acid in musts and wine, but from a practical point of view its use is very limited because it is poorly soluble in water (6.3 g/L at 25°C) although it improves in wine due to the presence of alcohol (54.5 g/L at 29.7°C in ethanol) although it will never reach values comparable to those of tartaric acid.
Due to these peculiarities and its easy availability, the use of fumaric acid in the wine industry is spreading rapidly, and consequently its presence in different wines for analysis. Here its determination as well as its impact on other oenological parameters such as alcohol, organic acids and taste become of primary interest and study. Based on a number of observations by technicians in the field, it was found that “fumaric” affects the determination of several chemical parameters of wines by FTIR analysis. Specifically, in the following study, alterations were observed in the determination of malic acid, total acidity, tartaric acid and volatile after addition of different doses of “fumaric.” The instrument used for this study uses FTIR technology and was purchased from Steroglass (i.e. Bacchus).
MATERIALS AND METHODS
Sample preparation
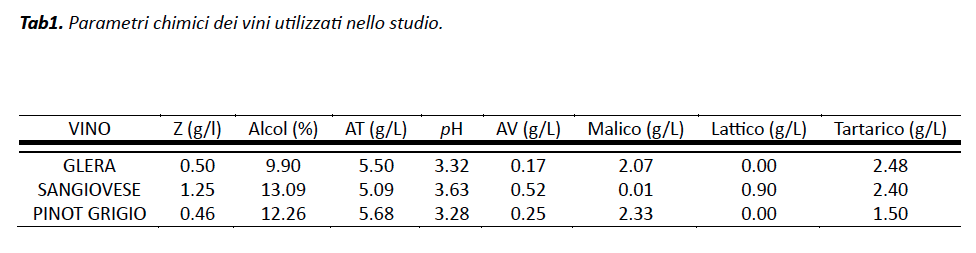
Three different types of wine were used for the study: a Glera, a Sangiovese and a Pinot Grigio; Their chemical parameters, obtained by FTIR analysis, are shown in TAB 1.
For the preparation of the concentrated suspension of fumaric acid, 200mg of acid was dissolved in 100mL of model wine (10% ethanol and 1% citric acid) in order to obtain a concentration of 200gr/HL, the suspension was stirred for 10′ at a temperature of 50°C to increase the solubilization of the acid.
In the preliminary test, increasing doses of “fumaric” were added to 50mL of Glera and Sangiovese in order to obtain a final concentration of 0-20-40-60gr/HL, after which the samples were shaken gently for 30′ at room temperature (20-25°C), filtered through a syringe filter (0.45μm) and finally defatted and loaded onto the Bacchus for analysis (about 10mL). Once the preliminary study was carried out, the following were prepared
Pinot Grigio samples (100mL) at increasing concentrations of “fumaric”: 10,20,30,40,50,60,70,80,90, 100 gr/HL plus the as-is (TQ), obviously free of fumaric acid. Each individual preparation was divided into halves (50mL): one half was analyzed immediately, the other half was kept in cold storage at 8°C for 7 days and finally analyzed.
The preparation procedure for the analysis was the same as that used for the preliminary test.
Analysis procedure
The instrument available in the company uses FTIR technology and consists of 4 separate units. The main unit, which constitutes the interferometer, is the most important part of the entire instrument as it is the one in which the laser beam that passes through the wine sample analysis cell is generated; the second unit consists of the hydraulic part that allows the recirculation of the sample inside the instrument; the third unit consists of the sampler equipped with a mechanical arm with a hollow needle for liquid aspiration and a 110-well rotor on which the test tubes for analysis are inserted; finally, the last unit is the computer in which the software for processing the spectra is installed. Depending on the type of sample matrix: must, which is particularly dense due to the high presence of sugars and the absence of alcohol or fermenting must or wine, the software allows the spectra to be processed through the Fourier transform and thus obtain numerical data for the main wine parameters of interest such as alcohol, sugars, lactic and malic acid, etc.
The procedure of analysis is quite simple; in fact, the most important aspect lies in the preparation of the sample itself. There are three main aspects to consider: The carbon dioxide content, which can affect the processing of spectra, the clarity of the sample, and the temperature.
Following this scheme, the wines used in this study were prepared as explained in the previous subchapter and then previously brought to room temperature (20-25°C), filtered (0.45μm) and finally degassed by hand or through a stirrer before being loaded onto the instrument. In this case, the samples used for analysis are essentially wines, so the protocol used is precisely the “Wine Method.”
RESULTS AND DISCUSSION
Preliminary study
FTIR is a technology that uses the infrared (IF) band and spectra interpretation software to analyze multiple parameters simultaneously using a single wine tube. It is now widely used in the oenological world because of its great speed of analysis and the possibility, through different types of samplers, to analyze several samples at once.
These characteristics are also no exception in this study, and it is possible to compare several samples at the same time and of consequence compare several parameters simultaneously.
In particular, in both Glera and Sangiovese samples, an increase in malic acid can be observed as the dose of fumaric acid increases. In Glera, respectively, it goes from +0.12 g/L at the lower dose to +0.79 g/L at the higher dose, although no more malic was added to the wine sample. Although less obvious, the same trend can also be observed on red wine, +0.04 g/L and +0.71 g/L, respectively (Chart 1). Given the chemical and physical characteristics of fumaric acid, which is also an organic acid like malic and tartaric acid, it can be assumed that once the sample in the cell is passed through the laser, fumaric emits a spectrum comparable or similar to that of malic acid, a proportional increase in value as a result of the (malic + fumaric) effect ensues. The influence of “fumaric” does not stop there, indeed it would seem that other parameters such as total acidity (although significant only in Sangiovese), volatile acidity and tartaric acid, are altered (data not shown). The latter shows opposite behavior to the other parameters, in fact decreasing rather than increasing as the dose of fumaric acid present increases: it goes from 2.48 g/L to 2.11 g/L in white wine and from 2.40 g/L to 2.04 g/L in red wine (data not shown). However, it is not possible to determine whether this effect is from a direct contribution of “fumaric” or an interpretation by “spectrum subtraction” by the instrument or software, caused by the increase in malic acid, since the two parameters are closely related.
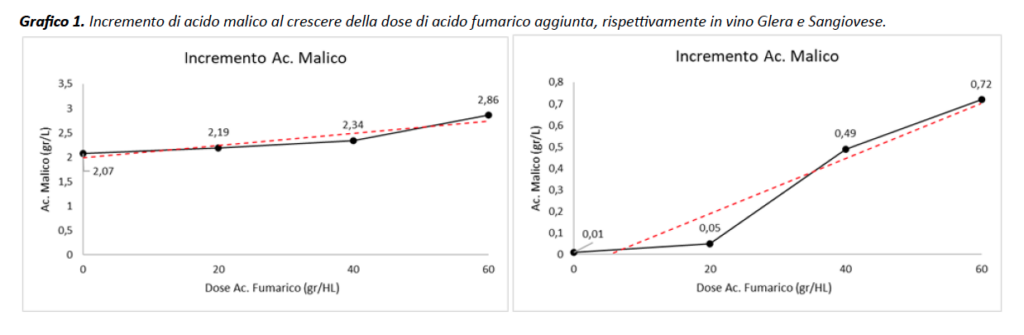
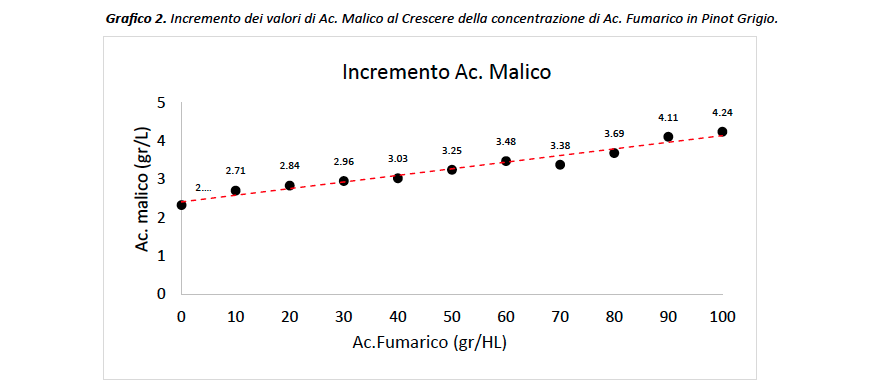
Conclusion Study
Once the preliminary study was completed, the investigation continued by going on to add more doses of fumaric acid to the wine, increasing from three points in the previous study to as many as ten in the current one. In this way, the body of information is broader and the effects of the organic acid under consideration can be further investigated. In contrast to the previous data, the effects of fumaric acid are appreciable already at the lowest dose, i.e., 10gr/HL, to almost double at the highest dose (Chart 2). Looking at graph 2, the ratio between the two acids appears to be directly proportional and very close to 1, that is, a concentration of 1 g/L of fumaric acid added corresponds to a proportional increase of 1 g/L of malic acid, in this case the value is 0.82. Furthermore, given the poor solubilizing power of fumaric acid we can assume that the actual concentration in wine does not exactly match the reported one, in fact it is more likely to be lower, so this Fumaric/Malic ratio (i.e. 0.82) is probably higher and really close to 1. This ‘information further supports the hypothesis that the two acids contribute equally in the spectrum of FTIR analysis of wines.
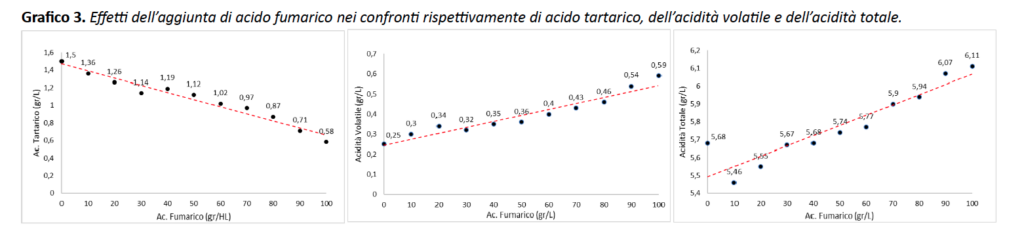
Regarding the other parameters examined such as total acidity, volatile acidity and tartaric acid, the effects of fumaric acid are much more important than those seen in the preliminary study (Chart 3). In particular, the effects on tartaric acid are more pronounced and it is possible to confirm an opposite behavior to that seen with malic acid, contrary also to those observed for total and volatile acidity, which show an increasing trend as the concentration of fumaric acid added to the wine increases. It must be emphasized, however, that this increase is not comparable to that which occurs for malic acid.
The final step involves the analysis of these samples after 5 days. The results are comparable to those obtained previously, so no discussion points emerged (data not shown).
CONCLUSION
Because of the quest for an increasingly healthier product with an ever-lower content of sulfites and other additives, fumaric acid represents a good opportunity to achieve this goal, given its ability to control malolactic fermentation and thus keep sulfur dioxide levels in wines low. Here comes the need to properly identify and quantify the content of this acid in our wines. Having a broad view of its effects on different wine parameters not only enables us to evaluate its relative effect, but also allows us to obtain consistently accurate and precise analytical results.
With this study we have highlighted how fumaric acid significantly influences several analytical parameters of wines, especially those closely related to organic acids with particular attention to malic and tartaric acids. Clearly, the technology used is only one of several techniques that can be used to determine these compounds, but it can lay the groundwork for improving and implementing a technology, FTIR, that is now a certainty in the wine industry. This study requires further investigation by going to work, for example, on the effect over time or by using other types of products or by going to investigate specifically the influence on infrared spectra with the aim of developing specific protocols that take into account the effects of fumaric acid that have emerged in this, but also in other similar studies.

